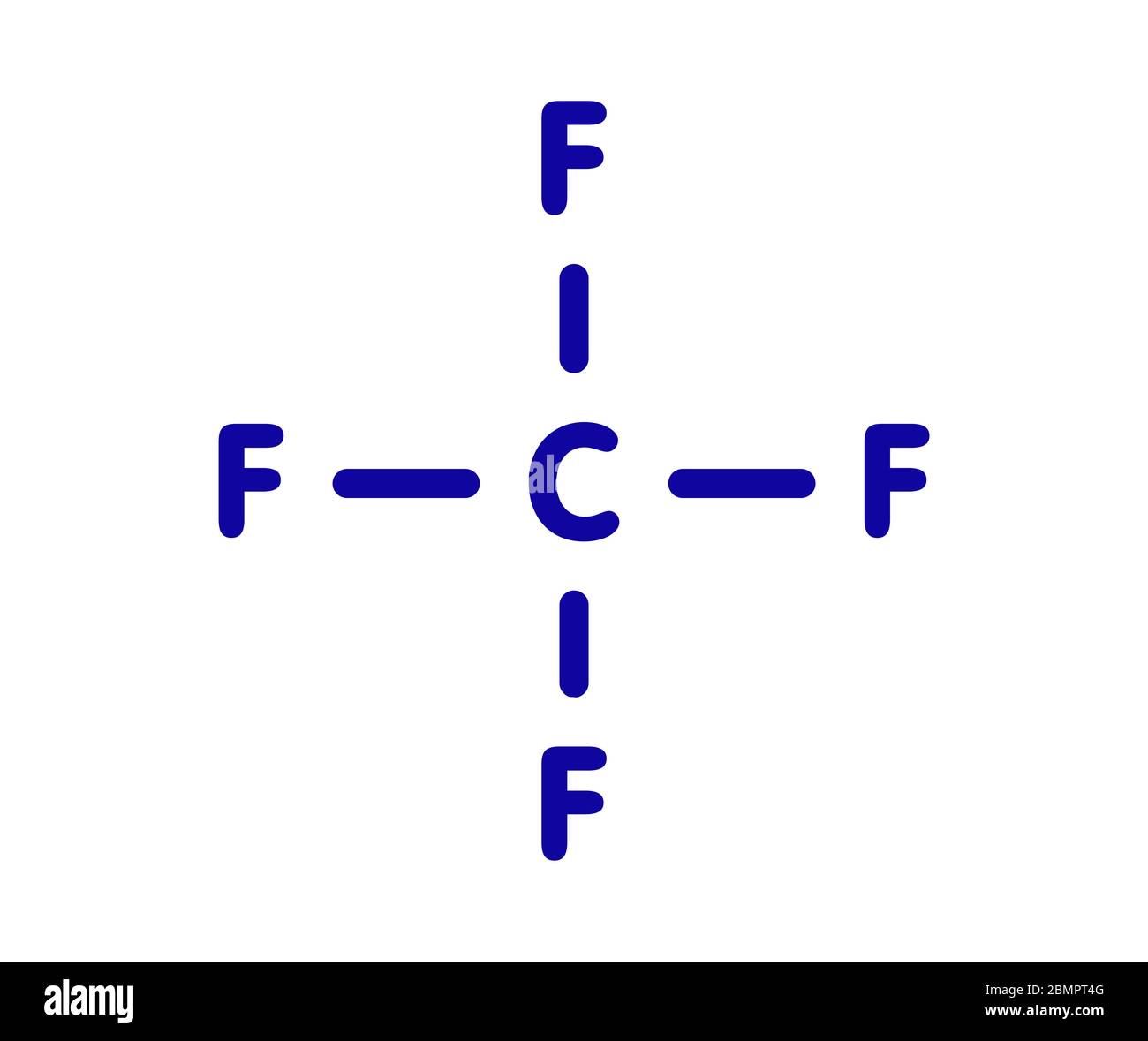
Carbon Tetrafluoride Formula Answered What Is The Chemical Formula Of
What is the Lewis dot structure for CF4? Question: What is the Lewis dot structure for C F 4? The Lewis Dot Structure: The Lewis dot structure for any molecule can be found by.

How to draw a CF4 Lewis Structure? Science Education and Tutorials
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

CF4 Lewis Structure Lewis Dot Structure for CF4Lewis Structure of
In the CF 4 Lewis structure, there are four single bonds around the carbon atom, with four fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. How to Draw The Lewis Structure for CF4 (Carbon Tetrafluoride) Watch on Contents Steps #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms

Draw the geometry of following compounds (i) SF4 (ii) xeF4 Chemistry
A three-step approach for drawing the CF4 Lewis Structure can be used. The first step is to sketch the Lewis structure of the CF4 molecule, to add valence electron around the carbon atom; the second step is to valence electron to the fluorine atom, and the final step is to combine the step1 and step2 to get the CCl4 Lewis Structure.

CF4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
As per the CF4 lewis structure, carbon has four atoms (fluorine) attached to it and no lone pair present on it. So, Hybridization number = 4 + 0 = 4 The hybridization number for CF4 is 4 which means it has Sp³ hybridization. Polarity Carbon tetrafluoride (CF4) has a central carbon atom surrounded by four fluorine (F) atoms.

How to draw a CF4 Lewis Structure? Science Education and Tutorials
The formula of CF4 molecular hybridization is as follows: No. Hyb of CF4 = N.A (C-F bonds) + L.P (C) No. Hy of CF4= the number of hybridizations of CF4. Number of C-Fbonds = N.A (C-F bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CF4 molecule.
SOLVED Explain how it is possible for CF4 to have polar bonds but be
Steps for Writing Lewis Structures. Calculate the sum of the valence electrons in the molecule. 1 C atom = 1 × 4 = 4 valence e -. 1 O atom = 1 × 6 = 6 valence e -. 2 Cl atoms = 2 × 7 = 14 valence e -. sum of valence e - = 24 valence e -. Construct a skeleton structure for the molecule. C is the central atom since it makes the most.
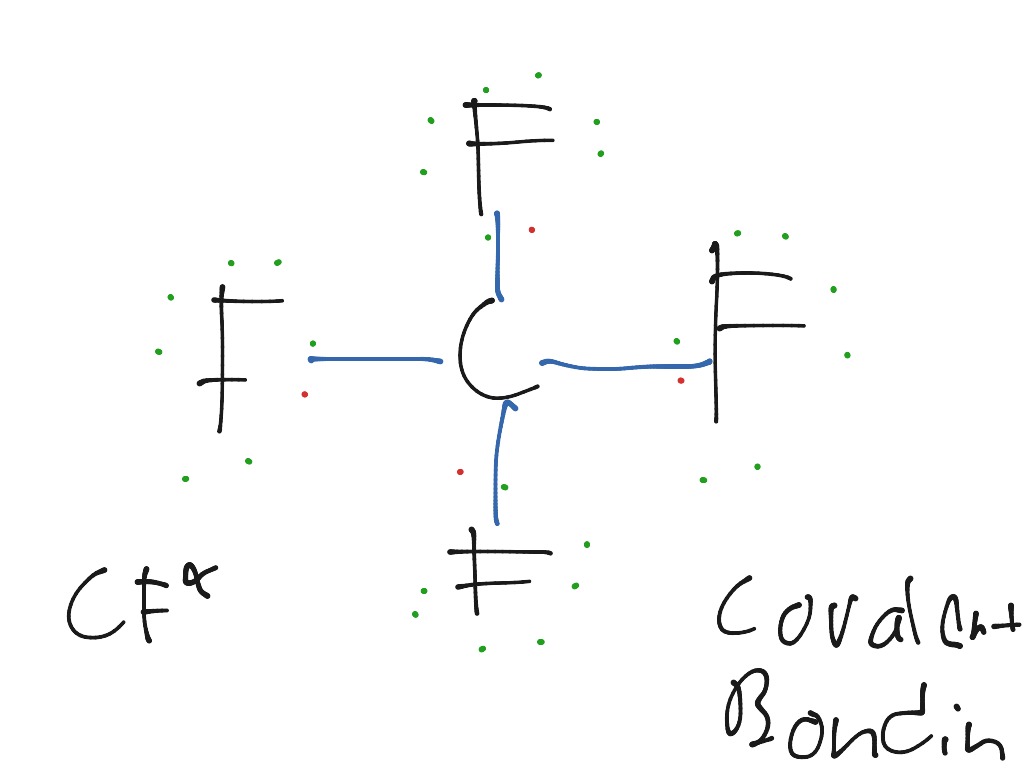
Lewis Structure Cf4
Written by Priyanka in Lewis Structure The chemical formula CF4 represents Carbon Tetrafluoride. It is also known as a Tetrafluoromethane (IUPAC name) and R-14 (owing to its use as a refrigerant). CF4 is the simplest perfluorocarbon (Hydrocarbons in which C-F bonds have replaced all the C-H bonds).
41+ Cf4 Molecular Geometry Bond Angles Tips GM
Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry of carbon tetrafluoride (CF4)
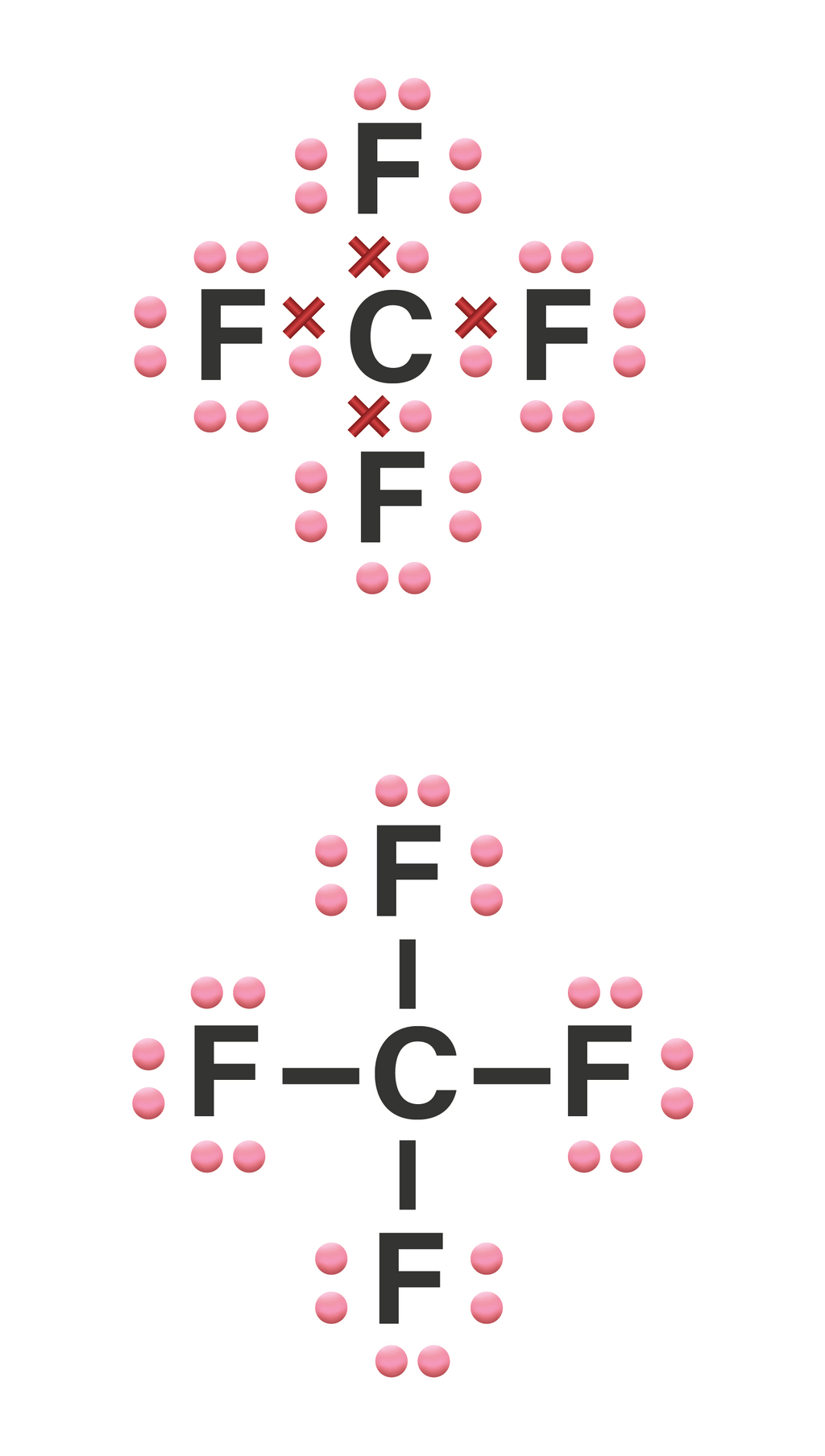
Tuliskan struktur Lewis dari molekul CF4!...
Lewis structure of CF4 contains four single bonds between the Carbon (C) atom and each Fluorine (F) atom. The Carbon atom (C) is at the center and it is surrounded by 4 Fluorine atoms (F). The Carbon atom does not have a lone pair while all four fluorine atoms have three lone pairs each.
Cf4 Science ShowMe
What is the Lewis structure of [//substance:cf4//]? Natural Language Math Input Extended Keyboard Examples Random Assuming completion date | Use opening date instead Using closest Wolfram|Alpha interpretation: Lewis structure Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals.
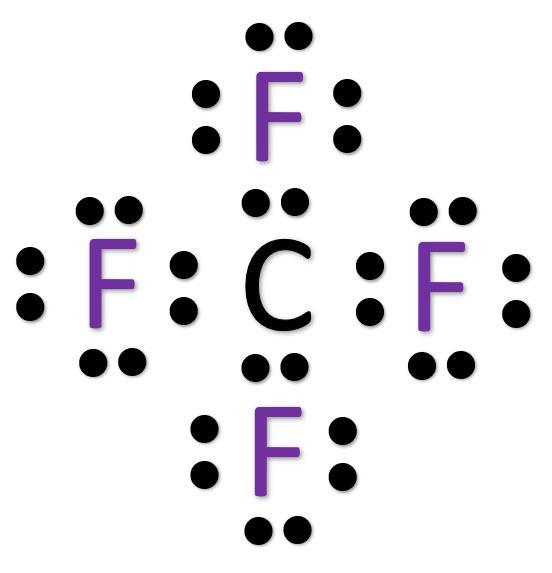
Create the Lewis structure of CF4. NOTE do only the step asked for in
Tetrafluoro methane, CF4 is a haloalkane or halo methane formed by replacing four hydrogen atoms of methane with fluorine atoms. It is a colorless, odourless gas which is used as refrigerant. It is an infalammable gas. How to draw Tetrafluoromethane, CF4 Lewis structure ?

Lewis Structure Carbon Tetrafluoride Cf4 Stock Vector (Royalty Free
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
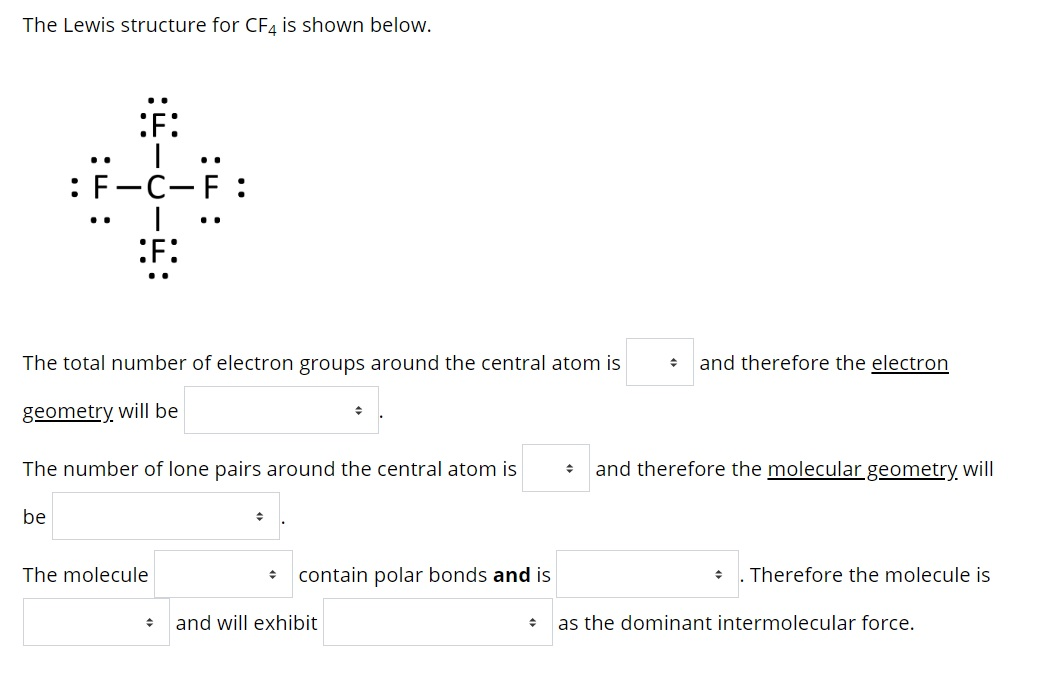
Solved The Lewis structure for CF4 is shown below. F
Now, let's learn to draw the Lewis structure of carbon tetrafluoride (CF4) with steps: Step 1: Find the total number of valence electrons each carbon tetrafluoride (CF4) molecule has: It is 32 as 4 are coming from the carbon atom and 7 are coming from each fluorine atom.

CF4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
How to Draw The Lewis Structure for CF4 (Carbon Tetrafluoride) Watch on So from the above diagram we have come to know that the CF4 molecule has four C-F bonds. Now in the next step we have to check whether these four C-F bonds are polar or nonpolar. Step #2: Check whether individual bonds are polar or nonpolar
[Solved] Draw the Lewis structure for CF4 , and indicate both the
122 15K views 3 years ago An explanation of the molecular geometry for the CF4 (Carbon tetrafluoride) including a description of the CF4 bond angles. The electron geometry for the Carbon.